Matsakaicin bambanci tsakanin nitrate da nitrite shine nitrite zangon oxygen da aka haɗa da zarra na nitrogen yayin da nitirin oxygen da aka haɗa da zarra na oxygen.
Dukansu nitrate da nitrite sune ƙa'idodi na ciki sun ƙunshi nitrogen da oxygen atoms. Duka iri biyu suna da cajin lantarki. Suna faruwa kamar man fari na kayan gishiri. Akwai wasu bambance-bambance tsakanin nitrate da nitrite; Zamu tattauna wadannan bambance-bambance a cikin wannan labarin.
Menene nitrate?
Nitrate wani abu ne mai ban sha'awa da ke da sinadarin sinadarai No3-. Ita ce mai narkewa mai ban mamaki wanda yake da kwayar zarra 4; daya nitrogen zarra da zarra uku na oxygen. Annion yana da -1 gaba ɗaya caji. Motar ruwa na wannan anion shine 62 g / mol. Hakanan, wannan anion an samo shi ne daga conjugate acid; nitric acid ko hno3. A takaice dai, nitrate shine tushen conjugate na nitric acid.
A takaice, nitrate ion yana da atom ɗaya nitrogen a cikin cibiyar da ta yi makoki tare da tatin oxygen uku ta hanyar haɗin sunadarai. A lokacin da la'akari da sinadaran sinadarai wannan anion, yana da iri daya babu guda uku (bisa ga tsarin tsayayyen gina antion). Saboda haka, ilimin lissafi na kwayoyin shine tsarin trigonal. Kowane atom oxygen yana ɗaukar cajin A - 2/3, wanda yake ba da cajin gona kamar -1.
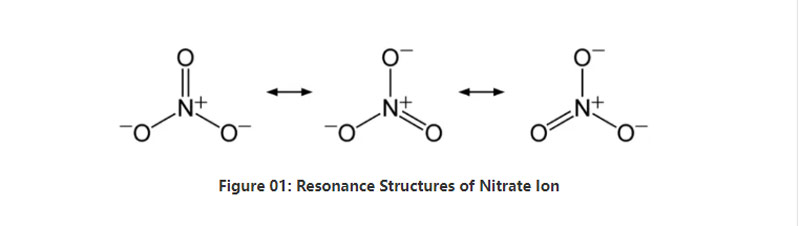
A daidaitaccen matsin lamba da zazzabi, kusan dukkanin labaran gishirin dauke da wannan bayyani ya narke cikin ruwa. Zamu iya samun dabi'un kayan ado na halitta a duniya a matsayin adibas; adiban ntratine. Yana da ya ƙunshi sodium nitrate. Bugu da ƙari, kwayoyin cuta na nitratia na iya samar da nitrate ion. Daya daga cikin manyan abubuwan amfani da salts masu nitrate yana cikin samar da takin mai magani. Bugu da ƙari, yana da amfani azaman wakili na oxidizing a cikin abubuwan fashewa.
Menene nitrite?
Nitrite gishirin ne mai gishiri da ke da sinadarai na sinadarai No2-. Wannan anion wani abu ne na symmetric, kuma yana da nitrogen na nitrogen wanda aka ɗaure zuwa ga tawayen oxygen biyu tare da abubuwa biyu da suka dace ba su da haɗin sunadarai guda biyu. Saboda haka, da nitrogen zarra yana tsakiyar kwayoyin. Annion yana da -1 gaba ɗaya caji.
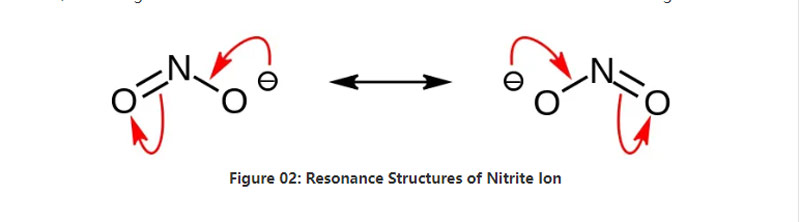
Mulkin molar na anion shine 46.01 g / mol. Hakanan, wannan antion an samo shi ne daga acid nitrous ko hno2. Saboda haka, shi ne conjug tushe na nitrous acid. Sabili da haka, zamu iya samar da jerin masana'antu na ado na ta hanyar wucewa ta hanyar samar da hydroxide mafita. Haka kuma, wannan yana samar da sodium nitrite wanda zamu iya tsarkake ta recrystallization. Bugu da ƙari, nitrite salts kamar sodium nitrite suna da amfani a cikin adana abinci saboda yana iya hana abinci daga haɓakar ƙwayar cuta.
Menene banbanci tsakanin nitrate da nitrite?
Nitrate wani anion ne wanda yake da sinadarai na sinadarai No3- Duk inda nitrite gishiri ne wanda yake da gishiri mai guba da yake da sinadarai No-. Don haka, banbanci na farko tsakanin nitrate da nitrite ya ta'allaka ne kan tsarin sunadarai na rigunan guda biyu. Wannan shine; Matsakaicin bambanci tsakanin nitrate da nitrite shine nitrite zangon oxygen da aka haɗa da zarra na nitrogen yayin da nitirin oxygen da aka haɗa da zarra na oxygen. Haka kuma, nitrate ion an samo shi ne daga conjugate na conjugate; Nitric acid, yayin da nitrika ion an samo shi ne daga acid nitrous. A matsayin wani muhimmin bambanci tsakanin nitrate da nitrite, zamu iya cewa wakili na oxidizing saboda yana iya yin kuskure kawai da nitrite.
Lokaci: Mayu-16-2022





