Babban bambanci tsakanin nitrate da nitrite shine nitrate yana ƙunshe da atom ɗin oxygen guda uku da aka haɗa da atom na nitrogen yayin da nitrite ya ƙunshi ƙwayoyin oxygen guda biyu da aka haɗa da atom na nitrogen.
Nitrate da nitrite duka anions ne na inorganic wanda ya ƙunshi nitrogen da oxygen atoms.Duk waɗannan anions suna da cajin lantarki -1.Sun fi faruwa a matsayin anion na gishiri mahadi.Akwai wasu bambance-bambance tsakanin nitrate da nitrite;za mu tattauna waɗannan bambance-bambance a wannan labarin.
Menene Nitrate?
Nitrate shine anion inorganic wanda ke da tsarin sinadarai NO3-.Anion polyatomic ne wanda ke da atom guda 4;nitrogen atom guda daya da oxygen atom guda uku.Anion yana da -1 gaba ɗaya cajin.Matsakaicin adadin wannan anion shine 62 g/mol.Har ila yau, wannan anion yana samuwa ne daga acid conjugate;nitric acid ko HNO3.A wasu kalmomi, nitrate shine tushen haɗin gwiwa na nitric acid.
A taƙaice, nitrate ion yana da zarra na nitrogen guda ɗaya a tsakiya wanda ke ɗaure da atom ɗin oxygen guda uku ta hanyar haɗin sinadarai.Lokacin yin la'akari da tsarin sinadarai na wannan anion, yana da nau'i guda uku NO bond (bisa ga tsarin resonance na anion).Don haka, ma'auni na kwayoyin halitta shine tsarin trigonal.Kowane atom oxygen yana ɗaukar cajin - 2⁄3, wanda ke ba da cikakken cajin anion azaman -1.
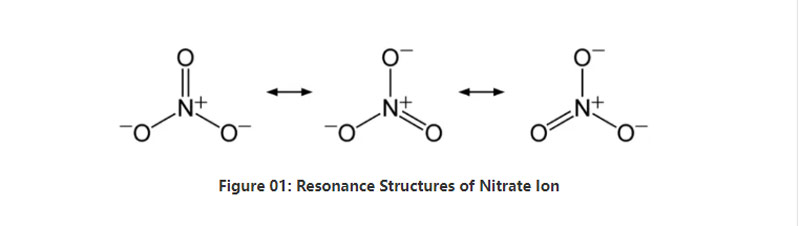
A daidaitaccen matsi da zafin jiki, kusan dukkanin mahadi na gishiri da ke ɗauke da wannan anion yana narkewa cikin ruwa.Zamu iya samun gishirin nitrate da ke faruwa a duniya a matsayin adibas;nitratine ajiya.Ya fi ƙunshi sodium nitrate.Haka kuma, kwayoyin nitrifying na iya haifar da ion nitrate.Daya daga cikin manyan amfani da gishirin nitrate shine wajen samar da takin zamani.Bugu da ƙari kuma, yana da amfani a matsayin wakili na oxidizing a cikin abubuwan fashewa.
Menene Nitrite?
Nitrite gishiri ne na inorganic wanda ke da tsarin sinadarai NO2-.Wannan anion simintin anion ne, kuma yana da zarra na nitrogen guda ɗaya wanda aka haɗa da atom ɗin oxygen guda biyu tare da NO covalent chemical bonds guda biyu.Don haka, atom ɗin nitrogen yana cikin tsakiyar kwayoyin halitta.Anion yana da -1 gaba ɗaya cajin.
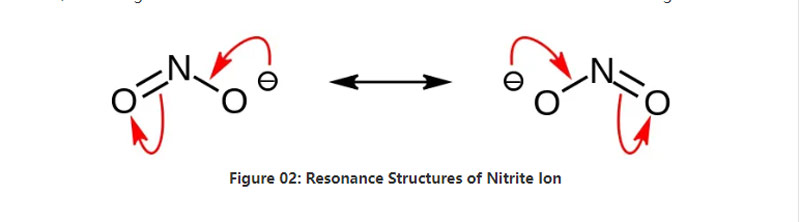
Girman molar na anion shine 46.01 g/mol.Hakanan, wannan anion an samo shi daga nitrous acid ko HNO2.Saboda haka, shi ne conjugate tushe na nitrous acid.Don haka, za mu iya samar da gishirin nitrite ta masana'antu ta hanyar wucewar tururi a cikin maganin sodium hydroxide mai ruwa.Bugu da ƙari, wannan yana samar da nitrite sodium wanda za mu iya tsarkakewa ta hanyar recrystallization.Bugu da ƙari, gishirin nitrite irin su sodium nitrite suna da amfani wajen adana abinci saboda yana iya hana abinci daga ci gaban ƙananan ƙwayoyin cuta.
Menene Bambanci Tsakanin Nitrate da Nitrite?
Nitrate anion inorganic anion yana da dabarar sinadarai NO3- yayin da nitrite gishiri ne na inorganic yana da dabarar sinadarai NO2-.Saboda haka, babban bambanci tsakanin nitrate da nitrite ya ta'allaka ne akan sinadarai na anions biyu.Wato;Babban bambanci tsakanin nitrate da nitrite shi ne cewa nitrate ya ƙunshi nau'in oxygen atom guda uku wanda aka haɗa da nitrogen atom yayin da nitrite ya ƙunshi nau'in oxygen guda biyu da aka haɗa da nitrogen atom.Bugu da ƙari, ion nitrate yana samuwa daga acid conjugate;nitric acid, yayin da nitrite ion aka samu daga nitrous acid.A matsayin wani muhimmin bambanci tsakanin nitrate da nitrite ions, zamu iya cewa nitrate wakili ne na oxidizing saboda yana iya jurewa kawai raguwa yayin da nitrite zai iya aiki a matsayin duka oxidizing da ragewa.
Lokacin aikawa: Mayu-16-2022





